China's State food and Drug Administration: 29 lots of drugs was found not qualified with leading pharmaceutical companies on the list (the list) | | food drug administration of medicines | unqualified _ news
State food and Drug Administration news, found in a recent national drug sampling, 25 drug companies produce 29 lots of drugs failed. Jilin people including Tang pharmaceutical and soothe the heart, Biao, Guangxi pharmaceutical compound danshen tablet, taiji group kechuanling granules. Batches of drugs has been ordered to stop production, recall, and accountability requires the rectification of this enterprise.
Circular points out that drug not eligible projects include property, identification, assay, and check under the weight differences, differences in weight, dissolution, dissolve, bacteria, acidity and colour effect, moisture, disintegration, loss on drying and microbial limits, etc.
Substandard batches of medicines
Korla Dragon of source drug industry limited responsibility company and Henan province Kang China arms celebrates drug industry limited production of batch for 20140902, and 20141201 and 140501 of soothe fill heart pills, Jilin people Hall drug industry limited production of batch for 20150301 of soothe fill heart tablets, Guangxi world PUMA drug industry limited production of batch for 150601 of compound Salvia tablets, Tai Chi group Chongqing medicine II factory limited production of batch for 1401003 of children cough spirit particles, Pharmaceutical company, Shaanxi Hua long xiaoer tuire oral liquid with the production lot number 20150101.
On the list are as follows:
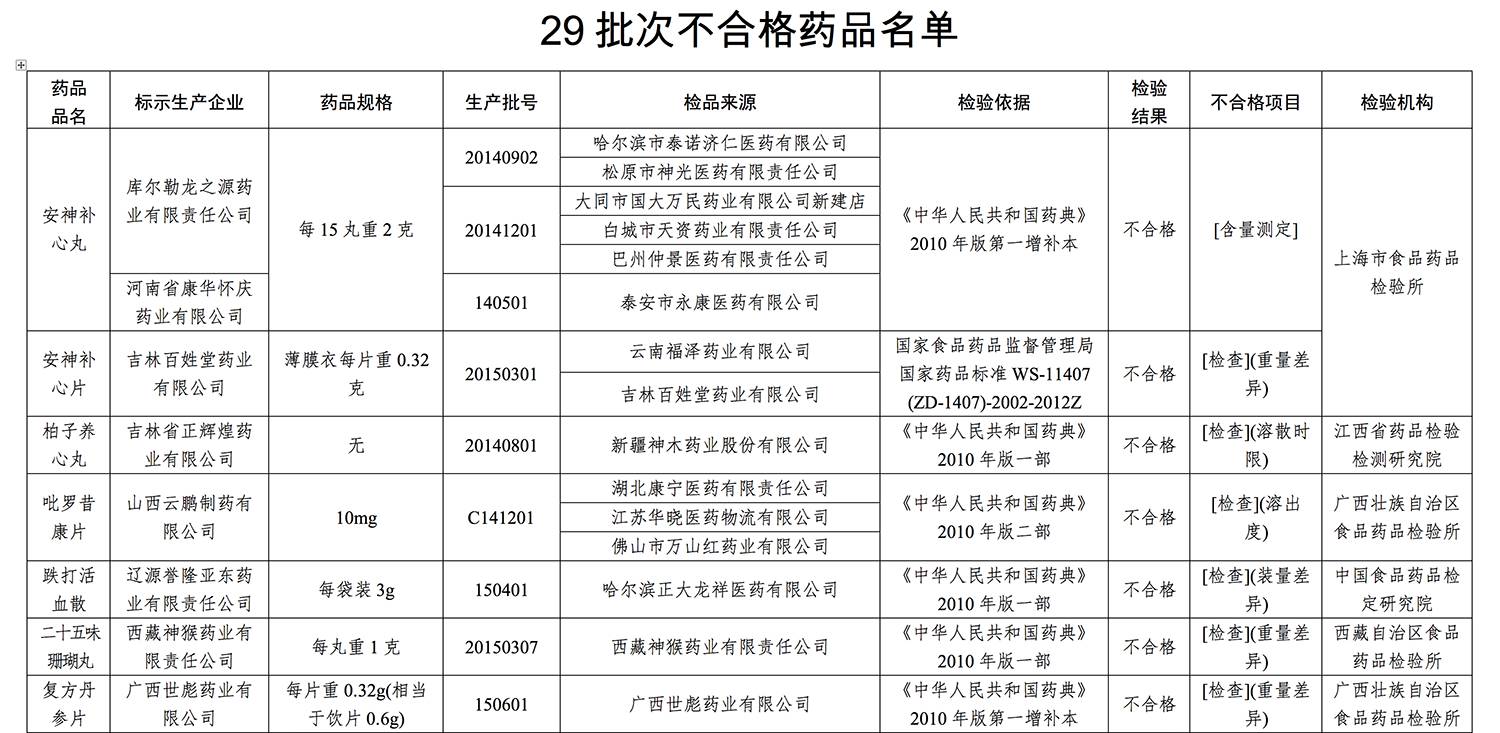
>
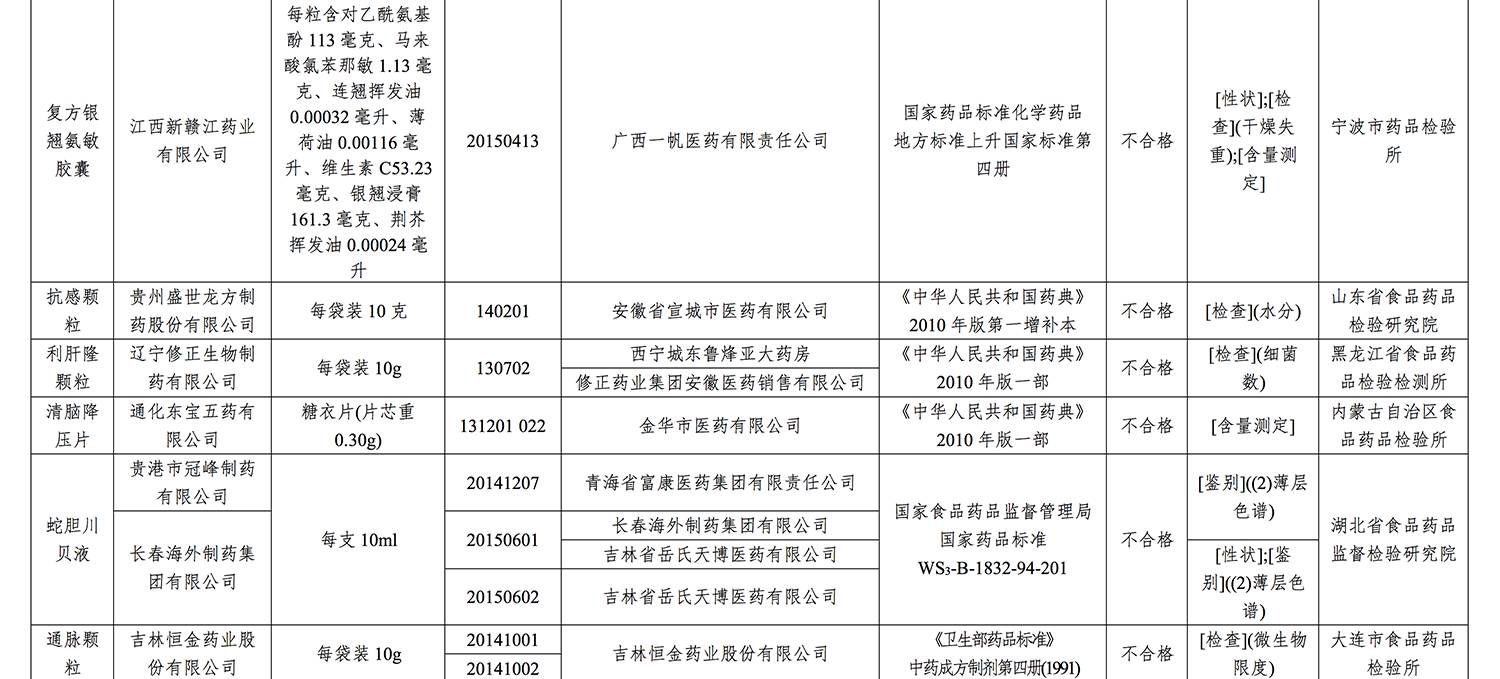
>
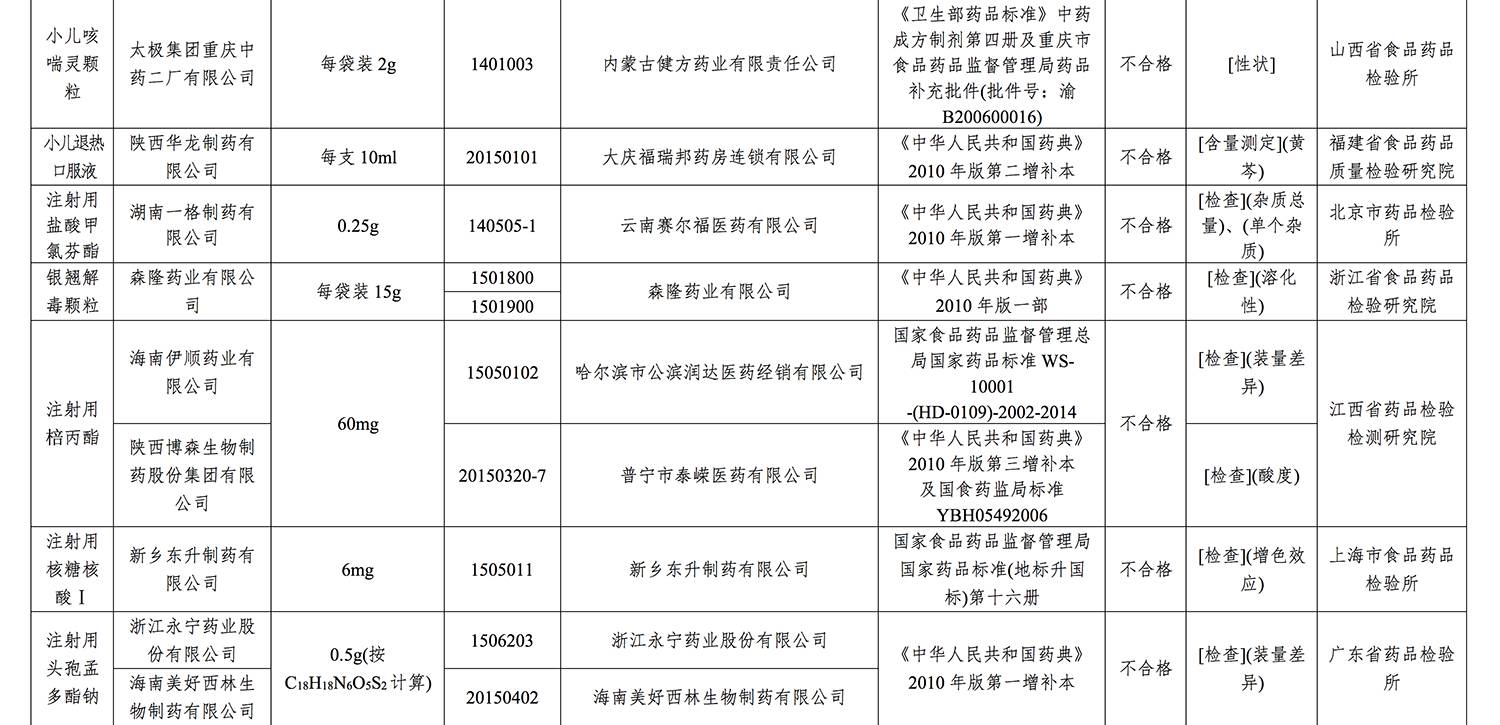
>

>
It is reported that these substandard drugs, provincial food and Drug Administration has ordered companies to suspend production and sales, product recalls and other measures. Food and Drug Administration require the food and Drug Administration to investigate the above enterprises according to the relevant provisions, according to the investigation, the law; and ordered the above enterprises thoroughly investigate the causes for quality problems and take targeted measures for rectification.
>: Tang Zheng Xueyou
Article keywords:Drug food and Drug Administration rejected drug companies
I want feedback
Save a Web page
CCTV news
中国国家食药监总局:29批次药品被认定不合格 有知名药企上榜(名单)|药品|食药监总局|不合格_新闻资讯
国家食药监总局消息,近期在国家药品抽验中发现,25家药企生产的29批次药品不合格。包括吉林百姓堂药业的安神补心片,广西世彪药业的复方丹参片,太极集团的小儿咳喘灵颗粒等。相关批次药品已被责令停产、召回,并对上述企业追责要求整改。
通告指出,药品不合格项目包括性状、鉴别、含量测定,以及检查项下的装量差异、重量差异、溶出度、溶化性、细菌数、酸度、增色效应、水分、溶散时限、干燥失重和微生物限度等。
不合格批次药品有
库尔勒龙之源药业有限责任公司和河南省康华怀庆药业有限公司生产的批号为20140902、20141201和140501的安神补心丸,吉林百姓堂药业有限公司生产的批号为20150301的安神补心片,广西世彪药业有限公司生产的批号为150601的复方丹参片,太极集团重庆中药二厂有限公司生产的批号为1401003的小儿咳喘灵颗粒,陕西华龙制药有限公司生产的批号为20150101的小儿退热口服液等。
具体名单如下:

>

>

>

>
据悉,对上述不合格药品,相关省份食品药品监督管理局已采取责令企业暂停生产销售、召回产品等措施。食药监总局要求有关地方食品药品监督管理局对上述企业依据相关规定进行查处,根据情况立案调查,依法处理;并责令上述企业彻查药品质量问题原因,采取有针对性的措施进行整改。
>责任编辑:郑学友
文章关键词: 药品 食药监总局 不合格 药企
我要反馈
保存网页
央视新闻